![]() By: Shree1news, 08 AUG 2021
By: Shree1news, 08 AUG 2021
India has granted Johnson and Johnson’s single-dose Covid-19 immunization crisis use authorisation, Union health minister Mansukh Mandaviya said on Saturday, making it the fifth antibody and the second foreign-made shot to be cleared for use in the country.
The drug organization, be that as it may, said it was too soon to give a delivery timeline for the vaccine. It said earlier on Friday that it applied for emergency use approval. The vaccine will be brought to India through a supply agreement with homegrown vaccine maker Biological E Ltd, J&J has said.
“India expands its vaccine basket! Johnson and Johnson’s single-dose COVID-19 vaccine is given approval for Emergency Use in India. Now India has 5 EUA vaccines. This will further boost our nation’s collective fight against #COVID19,” the Union health minister tweeted on Saturday.
Aside from the J&J immunization, the four other Covid-19 shots approved for emergency use in India are Bharat Biotech’s Covaxin, Serum Foundation of India’s Covishield, the Russian-made Sputnik V, and the Moderna vaccine.
“While we look forward to meeting our delivery commitments, it is premature for us to speculate on the timing of our vaccine deliveries,” the company told Reuters.
The J&J vaccine, which has shown 66-72% adequacy in various preliminaries, has been endorsed by controllers in the US, UK, Europe and the World health Organisation. The organization in May applied for an authorization to do bridging trials – more modest clinical preliminaries that are intended to evaluate if the discoveries are like bigger preliminaries completed somewhere else. In any case, it pulled out the solicitation last week and petitioned for crisis endorsement straightforwardly, utilizing an adjustment of strategy in India that permits Covid vaccine producers to do as such on the off chance that they have received approvals by certain regulators around the world.
In an statement gave on Saturday, the American pharma major said: “We are pleased to announce that on 7th August 2021, the Government of India issued Emergency Use Authorization (EUA) for the Johnson & Johnson COVID-19 single-dose vaccine in India, to prevent COVID-19 in individuals 18 years of age and older.”
“This decision was based on topline efficacy and safety data from the Phase 3 ENSEMBLE clinical trial, which demonstrated our single-shot vaccine was 85 percent effective in preventing severe disease across all regions studied, and showed protection against COVID-19 related hospitalization and death, beginning 28 days after vaccination. This is an important step forward in accelerating availability of our COVID-19 vaccine to help end the pandemic,” the company said.
The organization applied for the grant of emergency use authorisation to the focal medications standard control association (CDSCO) on Thursday.
“On 5th August 2021 Johnson & Johnson Pvt. Ltd applied for Emergency Use Authorization (EUA) of its single-dose COVID-19 vaccine to the Government of India. This is an important milestone that paves the way to bringing our single-dose COVID-19 vaccine to the people of India, and the rest of the world, through a collaboration with Biological E. Limited,” the company’s India spokesperson said on Friday.
The vaccine is developed by the Janssen Pharmaceutical Companies of Johnson & Johnson, to prevent the viral infection in individuals 18 years of age and older.
In April this year, the announced that Coronavirus immunizations cleared for use in western nations and Japan will get quick track endorsement in India, opening up the market for potential imports of Pfizer, Moderna and J&J dosages.
The sped up endorsement applies to dosages approved by the US’s Food and Drug Administration (FDA), the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), European Medicines Agency (EMA), Pharmaceuticals and Medical Devices Agency (PMDA) Japan or by WHO.
The US FDA approved J&J vaccine for emergency use in February 2021.
Source:A-N, HT
visit at: www.shree1news.com






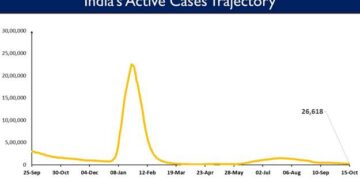
 Finance
Finance