New Delhi: The Drugs Controller General of India (DCGI) has approved Serum Institute of India’s proposal to manufacture a vaccine against omicron variant of coronavirus for examination, test and analysis, official sources said on Monday.
“With reference to your application, please find herewith the permission to manufacture SARS-CoV-2 rS Protein (COVID-19) recombinant spike protein nanoparticle vaccine (omicron variant) for examination, test and analysis under the provisions of New Drugs and Clinical Trials Rules, 2019 to manufacture the test batches of the drug/drugs mentioned therein,” an approval order issued on February 4 stated.
Director, Government and Regulatory Affairs at the SII, Prakash Kumar Singh had put in an application to the DCGI on January 6 stating the Pune-based firm, in collaboration with Novavax Inc, is working on the development of a vaccine against omicron, an official source said, adding that the SII has obtained permission and licence to manufacture SARS-CoV-2rS drug substance for examination, test and analysis.
“… the new coronavirus variant ‘omicron’ has already been reported in more than 60 countries and is spreading very fast worldwide and in our country also. Our CEO, Dr Adar C Poonawalla is very concerned about the protection of citizens of our country and world at large against coronavirus and its new variants and we are relentlessly working on development of SARS-CoV-2 rS Protein (COVID-19) Recombinant Spike Nanoparticle Vaccine (omicron variant).

SII has obtained permission and licence to manufacture SARS-CoV-2rS drug substance for examination, test and analysis. (Photo: File)
New Delhi: The Drugs Controller General of India (DCGI) has approved Serum Institute of India’s proposal to manufacture a vaccine against omicron variant of coronavirus for examination, test and analysis, official sources said on Monday.
“With reference to your application, please find herewith the permission to manufacture SARS-CoV-2 rS Protein (COVID-19) recombinant spike protein nanoparticle vaccine (omicron variant) for examination, test and analysis under the provisions of New Drugs and Clinical Trials Rules, 2019 to manufacture the test batches of the drug/drugs mentioned therein,” an approval order issued on February 4 stated.
Director, Government and Regulatory Affairs at the SII, Prakash Kumar Singh had put in an application to the DCGI on January 6 stating the Pune-based firm, in collaboration with Novavax Inc, is working on the development of a vaccine against omicron, an official source said, adding that the SII has obtained permission and licence to manufacture SARS-CoV-2rS drug substance for examination, test and analysis.
“… the new coronavirus variant ‘omicron’ has already been reported in more than 60 countries and is spreading very fast worldwide and in our country also. Our CEO, Dr Adar C Poonawalla is very concerned about the protection of citizens of our country and world at large against coronavirus and its new variants and we are relentlessly working on development of SARS-CoV-2 rS Protein (COVID-19) Recombinant Spike Nanoparticle Vaccine (omicron variant).
“Development of this vaccine shall be another example of vaccine production strength of our country in line with the clarion call of our prime minister ‘Making in India for the World’ and shall further keep our country’s flag flying high globally,” Mr. Singh had stated in the application,” the source said.
Source:IA






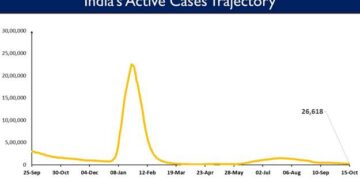

 Finance
Finance