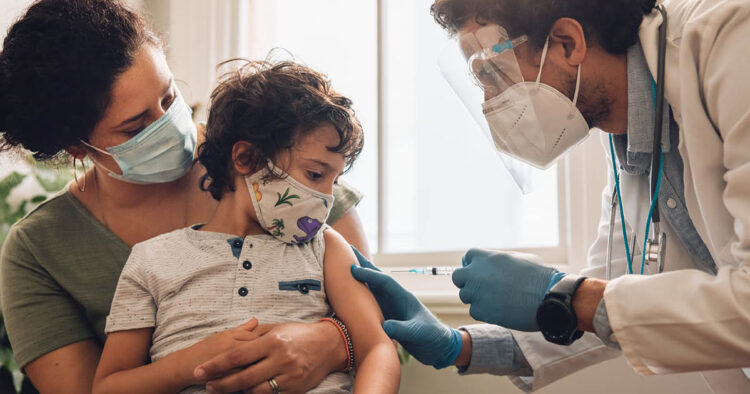
The US Food and Drug Administration (FDA) has approved the use of the Moderna and Pfizer-BioNTech Covid-19 vaccines for children as young as 6 months, marking the first time that these vaccines have been approved for use in this age group.
The FDA amended the emergency use authorization (EUA) for the Moderna vaccine on Friday to include use of the jab in individuals 6 months to 17 years of age, according to Xinhua news agency.
Previously, the vaccine was only available to people over the age of 18.
The FDA amended the EUA for the Pfizer-BioNTech vaccine to include use of the jab in individuals aged 6 months to 4 years.
The vaccine had previously been approved for use in people aged 5 and up.
The FDA stated that its evaluation and analysis of these vaccines’ safety, effectiveness, and manufacturing data was rigorous and comprehensive, supporting the EUAs.
The agency determined that the known and potential benefits of the Moderna and Pfizer-BioNTech Covid-19 vaccines outweigh the known and potential risks in the pediatric populations for which each vaccine is approved for use.
The Moderna vaccine is given in a two-dose primary series, one month apart.
The Pfizer-BioNTech vaccine is given in a three-dose series, with the first two doses given three weeks apart, followed by a third dose given at least eight weeks after the second dose.
The FDA made its decision following a meeting of its advisors earlier this week, during which they voted to recommend the two vaccines.
According to Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, the advisory committee meeting was part of a transparent process to help the public understand the safety and effectiveness data supporting the authorization of these two vaccines for paediatric populations.
“Those trusted with the care of children can have confidence in the safety and effectiveness of these Covid-19 vaccines and can be assured that the agency was thorough in its evaluation of the data,” said FDA Commissioner Robert Califf in a statement.
On Saturday, the US Centers for Disease Control and Prevention (CDC) will hold a meeting of advisors to vote on guidelines for pharmacies and doctor’s offices to administer the shots.
Before children can begin receiving shots, CDC Director Rochelle Walensky must sign off on the guidelines.
Vaccinations are expected to begin as soon as June 21.
Appointments may be limited at first, but according to Ashish Jha, who oversees the Biden administration’s Covid-19 response, every parent who wants to get their child vaccinated should be able to do so within weeks.






 Finance
Finance