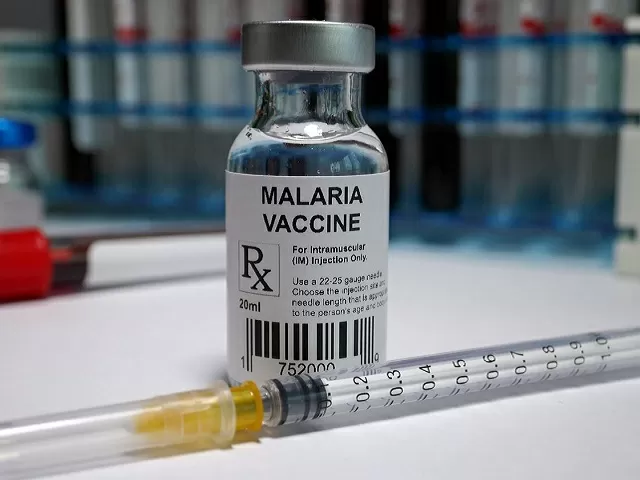
The World Health Organization (WHO) has announced their recommendation for a pioneering second malaria vaccine known as R21/Matrix-M, which has a 75% efficacy rate.
The University of Oxford and the Serum Institute of India collaborated to develop this vaccine with the intention of safeguarding vulnerable youngsters who are at risk of contracting this deadly disease.
Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, expressed his satisfaction during a press conference in Geneva.
“Today, it gives me great pleasure to announce that WHO is recommending a second vaccine called R21/Matrix-M to prevent malaria in children at risk of the disease,” he said, as quoted by Reuters.
Tedros further stated that the R21/Matrix-M vaccine would be available for use by mid-2024. He announced plans to economically price the vaccination doses, ensuring widespread accessibility, with costs ranging from $2 to $4.
Furthermore, the WHO will review the vaccine for prequalification, confirming its approval, allowing global organizations like as GAVI and UNICEF to obtain the vaccine from producers.
The Serum Institute of India emphasized the substantial research and testing that backed up this judgment.
“The recommendation was based on pre-clinical and clinical trial data which showed good safety and high efficacy in four countries, at sites with both seasonal and perennial malaria transmission, making it the world’s second-ever WHO-recommended vaccine for preventing malaria in children,” they stated in a released statement.
Dr. Lisa Stockdale, Senior Immunologist at the University of Oxford’s Jenner Institute, stated that researchers need to conduct further studies. These studies aim to not only confirm the vaccine’s efficacy but also to gain a deeper understanding of its mechanisms. This knowledge will then be applied to future vaccination efforts.
Adar Poonawalla, the CEO of the Serum Institute of India, recognising the long-standing threat that malaria poses to global populations, said, “For far too long, malaria has threatened the lives of billions of people across the globe, disproportionately affecting the most vulnerable amongst us.”
With R21/Matrix-M entering the stage, it will contend with GSK Plc’s RTS,S, which the UN agency approved in 2021 and is currently available under the brand Mosquirix.
Prior to WHO clearance, Ghana was the first country to start the vaccine in children aged 5-36 months, the age group most at risk of malaria death.
Source:IT







 Finance
Finance